Results of ETINCEL-OFDT survey on electronic cigarettes
Prevalence, purchase behaviour, usage, and motivation of e-cigarette users
(Original publication in French is available from OFDT website)
(Scroll down for a PDF version of this translation)
OFDT - Observatoire Français des Drogues et des Toxicomanies [French Monitoring Centre for Drugs and Drug Addiction]
Note n°2014-01: results from ETINCEL-OFDT survey (November 2013)
Saint-Denis, France, 12/02/2014
Aurélie Lermenier and Christophe Palle (OFDT - «Indicators» Unit)
Design and follow-up of the survey : Marie-Line Tovar («General Population Surveys» Unit) & Aurélie Lermenier
English translation by Jacques Le Houezec, translation notes are indicated within brackets [ ]
Presentation of the survey
While electronic cigarettes appear as a growing phenomenon in France over the last two years, data on prevalence and usage remain fragmentary and difficult to interpret due to the lack of information on the methodologies used. Being deeply involved in issues related to tobacco, for the last ten years the OFDT has produced monthly statistical reports and launched every year a detailed summary review. However, it has become increasingly difficult to interpret trends in the tobacco market without taking into account electronic cigarettes. This is why, at the end of 2013, OFDT decided to conduct a survey focusing on this product, with the aim to provide the government and professionals, within a short time frame, the first reliable estimate of this phenomenon, in order to measure its impact on tobacco for the 2013 annual report.
The ETINCEL-OFDT survey (telephone survey for information on electronic cigarettes) was conducted between 12 and 18 November 2013 among a representative sample of 2,052 individuals aged 15 to 75 years, from the metropolitan population (excluding Corsica). A land-line telephone data base (including numbers starting with 01 to 05 [old numbers denoting geographical areas, before introduction of cable and DSL connections] and 09 [non-geographical numbers, after introduction of cable and DSL], stratified by region and urban category) was randomly generated. The sample of individuals from this data base, interviewed by telephone during one week, was established by the method of quota sampling according to sex, age group, and occupational category. The results were calibrated to the latest data from the INSEE (Institut National de la Statistique et des Études Économiques [French National Institute for Statistics and Economic Studies]).
The questionnaire consisted of 17 questions (see Annex 1). It addresses the issues of awareness of electronic cigarettes, frequency of use, purchase behaviour of electronic cigarettes and e-liquids or refills, user motivations, etc. Questions about tobacco use were also asked to the respondents in order to know the smoking status (e.g. current or ex-smoker) of electronic cigarette users and measure the potential impact on smoking prevalence.
This paper presents the main results of the ETINCEL-OFDT survey and puts them in perspective with other studies conducted in France.
A large majority of French people are aware of electronic cigarettes
In November 2013, almost nine out of ten French people (88% [86.8 to 89.6]) stated that they were aware, at least by name, of electronic cigarettes. In March 2012, the Eurobarometer on tobacco indicated that electronic cigarettes awareness in France was already significant, but three points below the European average of 66%. Awareness of this product was greater among young people aged 15 to 24 years (93%) and among managers and higher intellectual professions (93%) and was slightly lower among 65-75 years (83%), and therefore among the retired (85%). Awareness among smokers, who are the target audience for electronic cigarette marketing campaigns (presenting it more or less openly as a means of smoking cessation), was higher than in people who never or virtually never smoked (93% vs. 85%).
What is an electronic cigarette?
Developed in China in the mid-2000s, the electronic cigarette, also known as the e-cigarette mimics the feeling of a classic tobacco cigarette. There are two types : disposable (looks like a real cigarette) and rechargeable (AC or USB cable ; it then rather looks as a large pen). Rechargeable electronic cigarettes (the majority of the French market) consists of a battery, a clearomiser that contain a resistance and the e-liquid, and a tip that allow to inhale the vapour generated at the clearomiser. By pressing a button, the battery supplies the resistance with power which heats the e-liquid soaked by the wick of the clearomiser and turns it into vapour, which is inhaled by the user. The e-liquid is made of propylene glycol and/or vegetable glycerin, various flavours (tobacco, mint, apples, etc.), a small proportion of alcohol and/or purified water, and may contain or not nicotine at different concentrations.
|
Already one in five French people used it at least once
At the end of 2013, 18% [16.7-20.1] of people surveyed reported having used at least once an electronic cigarette. This is 2.5 times more than in March 2012, when the rate of experimentation in France was 7 % (identical to that in all countries of the European Union).3
Among those who had not yet tried electronic cigarettes, only a small minority (2.3% [1.6-3.0]) intended to do in the near future. This proportion of potential experimenters was twice as high among manual workers (4.9%), and five times higher among smokers (11.2%).
Rather young experimenters, and tobacco users
Men were more likely than women to have ever used electronic cigarettes (22% vs. 15%). The proportion of experimenters decreased as age increased (Figure 1) : nearly a third (31%) of 15-24 years-old had tried them, as opposed to no more than one in five of 35-44 years-old, and one in ten (9%) between 55-64 years-old. For reasons probably more related to age than professional status, retired people were less likely to try these products. Not surprisingly, being a smoker or having smoked in life influences the level of experimentation : half of smokers (51%) report having tried electronic cigarettes as opposed to only 12% in ex-smokers, and 3.5 % among respondents who never or rarely smoked. Thus, among the experimenters, three quarters were smokers, one in six was a former smoker and nearly one in ten (9% ) had never smoked or had only tried smoking. Furthermore, although the sample size makes it difficult to draw geographic comparisons, experimentation appears less common in the north (Nord-Pas-de-Calais: 7.9 %) than in the west (23,1% in the area consisting of the regions Brittany, Pays de la Loire, and Poitou-Charentes) and the South west (21,3% in Aquitaine, Limousin, and Midi-Pyrénées).
Electronic cigarette use concerns one in fifteen person in the last month
At the end of 2013, recent use (within the last thirty days and excluding experimentation) of electronic cigarettes concerned 6.0% [5.0-7.0] of French people, or a third of those who tried them. Although they were more likely to experiment than older people, 15-24 years-old were proportionately less likely to have used it in the month preceding the survey, followed by 25-34 years-old. It is after the age of 35 that people seemed more likely to "adopt" electronic cigarettes after having tried them (Figure 1): regardless of their age group, more than one experimenter in three reported recent use. It is likely that the trendy effect is playing more in young people experimentation, who would try it more by curiosity, while older users are more likely to use them specifically to reduce or stop tobacco use.
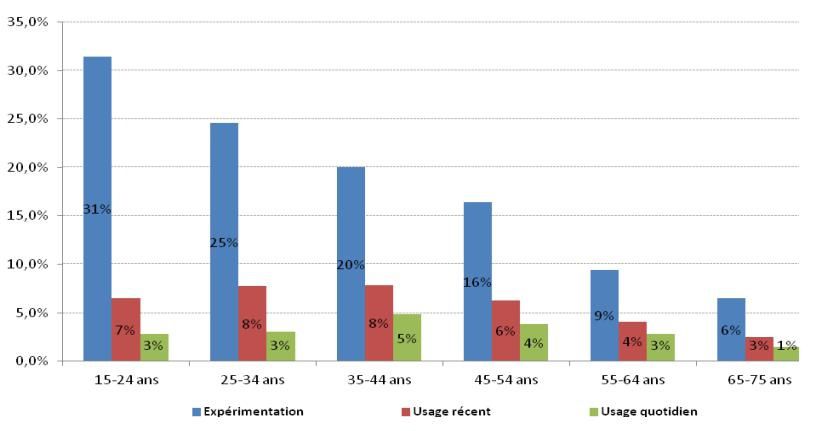
Figure 1 : Proportion of experimenters (blue bars), recent use (red bars), and daily use (green bars) of electronic cigarettes, by age group
Source : ETINCEL-OFDT survey (November 2013)
Unlike experimental use, more common in men, there was no gender difference in recent (and daily) use of electronic cigarettes. Apart from the lower proportion of retired people among users in the last month (3.1%), given their age, there was also no significant differences according to their socio-economic category. However, as for experimental use, use in the last month was higher in the West of the country (9.3%), and lower in the North (1.6%), possibly due to an easier access to cheaper tobacco in Belgium and Luxembourg.
All recent users of electronic cigarettes reported current tobacco use, or having used tobacco in their lifetime, but smokers were significantly more likely to use electronic cigarettes than ex-smokers (78% vs. 22%).
Slightly more than 3% of daily users
More than half (54%) of those who used electronic cigarettes in the last month use them daily, or 3.3% [2.5 to 4.1] of French people (Figure 2). As already observed for recent use, the difference between the youngest and the oldest users was confirmed for daily use: among recent users of electronic cigarettes, only 44% of 15-24 years-old used them daily, as opposed to 50-75% of 50-75 years-old. This seems to reinforce the hypothesis that young people would bow to fashion, whereas those being over 50 years-old are more likely engaged in giving up tobacco use or in harm-reduction, as soon as they try them, certainly in relation with being older. When faced with health damages, actual or perceived as very likely, caused by usually long-life (several decades) smoking, older smokers tend to turn to electronic cigarettes to reduce their risks.
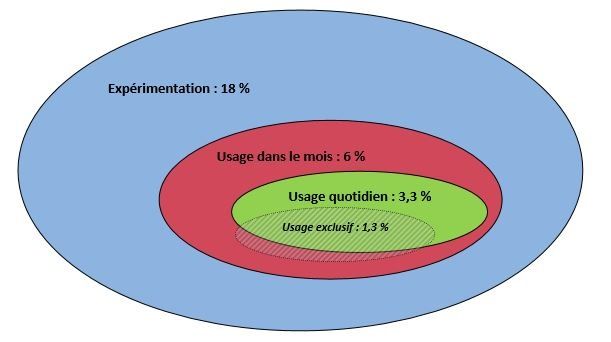
Figure 2 : Proportion of experimenters, recent users, and daily users of electronic cigarettes in France
Experimental use: 18 %, Use in the last month: 6 %, Daily use: 3.3 %, Exclusive use: 1,3%
Source : ETINCEL-OFDT survey (November 2013)
Many daily users of electronic cigarettes are still using tobacco, since two-thirds of them were dual users (tobacco and electronic cigarettes). However, among them, more than six in ten (62%) used "electronic cigarettes most of the time and tobacco sometimes"; a quarter of them responded the opposite.
Exclusive electronic cigarette users, i.e those who do not currently use tobacco, represented 1.3% [0.8-1.8] of the surveyed sample, and the vast majority (81%) used them daily.
A growing usage since spring 2013
Three quarters (76%) of vapers who used electronic cigarettes during the thirty days preceding the survey started using them less than six months ago, that is to say, since April-May 2013, which coincides with a period of extensive media coverage, particularly related to a report on the subject submitted by OFT to the Ministry health. Only 13% reported having started to use them more than one year ago.
The vast majority (78%) of those who used electronic cigarettes in the last month owned their own electronic cigarette, while 16% used those belonging to other people (the remainder sharing them with one other user: spouse, friend, etc.). This latter figure may be explained by the desire to test the product and the liquid flavours before making a purchase that represents a certain upfront investment: a minimum spend of 50 Euros for a rechargeable electronic cigarette and a little less than 6 Euros per 10 ml bottle of e-liquid. A lower proportion of vapers aged 15-24 owned their own electronic cigarette (44%), probably because they are often less likely to be regular users, while the proportion rose to 93% among 35-54 years-old.
Nearly a quarter (24%) of recent users reported not knowing the nicotine content of the liquid or refill for the electronic cigarette they used (the majority of these being people who did not own their own). Among those who did know, 11% reported using a 0 mg/ml concentration (no nicotine), highlighting the low proportion of non-nicotine-dependent users, or those who have successfully given up, after a gradual reduction in nicotine dosage. Four in ten vapers choose a medium dosage (between 7 and 12 mg/ml), while the remaining users were equally distributed (24%) between low dosage (between 1 and 6 mg/ml) and high ones (greater than 12 mg/ml).
Purchases mainly in speciality shops
The electronic cigarettes market is still poorly organized and regulated, and it is shared by many manufacturers and retailers. However, the majority of purchases by those surveyed (58%) were made in speciality shops for this type of product, even though the purchases in tobacconists [a monopoly for tobacco sales in France] was by no means negligible (21% - Figure 3). The Internet is a rather small source of supply: 9% of those surveyed bought their electronic cigarette online. Purchases from pharmacies [a monopoly for drugs in France, where there exist no GSL even for OTC drugs], which are actually prohibited to sale these products, or from supermarkets were mentioned, but concerned very few purchasers. Speciality shops were also the source of the majority of purchases of e-liquids and refills: 54% of those surveyed used them, whilst 24% did it in a tobacconist.
No matter where they purchased their electronic cigarette, a very large proportion of users used the same supply channel for purchasing refills. This was especially true for tobacconists (91%) but also for speciality shops (88%): although there is no evidence to establish whether this was in the same place, this figure may well suggest that users are attached to the personal relationship with a vendor who can advise them.
Finally, the market for disposable electronic cigarettes appears to be very small: only 4% of those who had used electronic cigarettes in the last month used these type of products, which are sold mostly for trial purposes, rather than of loyalty. Indeed, they are easy to use, similar to conventional cigarettes and allow to try the product at a low cost. It is likely that with the increase in electronic cigarettes use, disposables have experienced a decline in their market share.
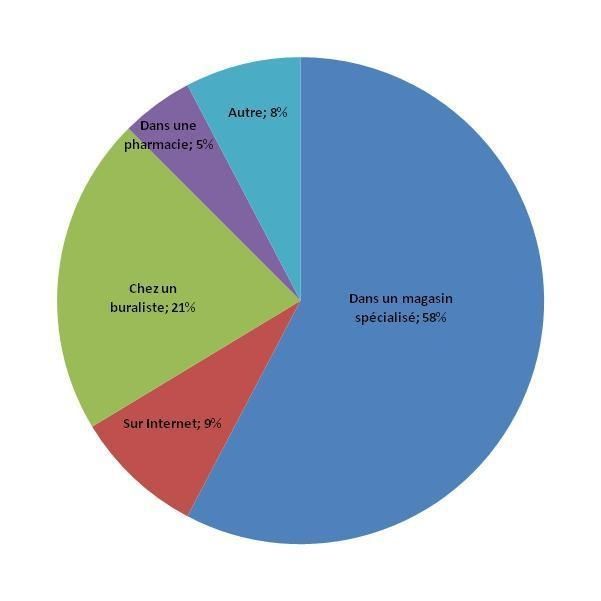
Figure 3: Distribution of locations where electronic cigarettes are purchased in France
Speciality shop 58%, Tobacconist 21%, The Internet 9%, Pharmacies 5%, Other places 8%
Source : ETINCEL-OFDT survey (November 2013)
Primary motivation : giving up completely
Half (51%) of people surveyed who reported simultaneous use of tobacco and electronic cigarettes spontaneously claimed that their main and ultimate goal was to stop completely the use of both products. Among the motivating factors reported, next came, but far behind, the reduction of tobacco consumption but without giving up entirely (11.5%), and then substituting electronic cigarettes for tobacco (8.2%), both of which might relate to a form of harm-reduction. Other users highlighted the decrease in health risks, reduction in tobacco inconveniences, cost, and the ability vape anywhere.
The product is therefore strongly linked to the idea of smoking cessation, and even beyond that to reduce or even to eliminate any nicotine dependence. According to a survey conducted in Great Britain among users of electronic cigarettes, the idea of smoking cessation was also the most common: 34% of vapers reported using electronic cigarettes to quit smoking, and 28% "because they had already tried to quit and wanted something to help to stop for good." They were 22% to want to reduce their consumption without giving up completely, and the same proportion to be motivated by the potential savings.
Among the very small proportion of surveyed people who were former smokers (even occasional ones) and had used electronic cigarettes within the last month (namely 1.2%), most of them (84%) considered they had completely stopped smoking by using them: this represents 1% of the French population. Without presuming the effectiveness of electronic cigarettes for smoking cessation, especially as the numbers here are very small, it seems that smokers are convinced of its usefulness for achieving this goal, as an alternative to nicotine replacement therapy and smoking cessation medications. This opinion is also shared by a significant part of the population: as 43% of French people believe that these products are an effective mean to reduce or stop smoking.
Conclusion
In November 2013, the vast majority of the French population had heard about electronic cigarettes, and between 7.7 to 9.2 million people had tried them, mainly young people and smokers. In the month preceding the survey, 6% of the population were using electronic cigarettes. Between 1.1 and 1.9 million people reported daily use of electronic cigarettes in France: some 67% of tobacco smokers used them mainly to stop or reduce their daily tobacco consumption, and therefore potentially the health risks associated with smoking. Although 9% of those using electronic cigarettes reported having never or rarely smoked tobacco, all the regular vapers were or had been smokers: electronic cigarettes appear therefore, at least for the moment, to rather be a way out of smoking, rather than a "gateway" into smoking.
The exclusive use of electronic cigarettes is fairly limited, but may increase over time, as smokers use these products to reduce their tobacco dependence. The ultimate goal of vapers is focused on complete cessation; with three quarters of regular users having started using electronic cigarettes less than six months before the survey, more time may be needed before effective cessation is confirmed, which at the end of 2013, represents 1% of the French population.
Rechargeable electronic cigarettes represent the vast majority of the market (over 95% of those who had vape in the month) and the purchase of the object itself and the refills are made primarily in speciality shops (over 50 %) and tobacconists (over 20 %).
Further surveys are needed to consolidate these findings and their evolution over time. At the beginning of 2014, the increased media attention and the strong momentum of the electronic cigarette market show no sign of slowing: it is therefore likely that the number of users, going from experimentation to daily use, will continue to rise.
Annex 1. Questionnaire
Survey on behaviour of use and purchase of electronic cigarettes and tobacco
Part I : Electronic Cigarette
Q1 - Have you heard, even if only by name, of electronic cigarettes, also known as “e-cigarettes”, “personal vaporisers” or “vaporette”?
1- Yes
2- No
Q2 - (if yes to Q1) Have you ever tried an electronic cigarette?
1- Yes
2- No
Q3 - (if no to Q2) Do you intend to try an electronic cigarette in the near future?
1- Yes - Go to Q10
2- No - Go to Q10
Q4 - (if yes to Q2) Have you used an electronic cigarette within the last 30 days (apart from for the first time)?
1- Yes
2- No - Go to Q10
Q5 - (if yes to Q4) How often do you use electronic cigarettes?
Interviewer: List responses
1- Every day
2- Several times a week
3- Once a week
4- Two to three times a month
5- Once a month
6- (Don’t know)
Q6 - (if yes to Q4) When did you start using electronic cigarettes?
Interviewer: List responses
1- Within the last 30 days
2- Between 1 and 6 months ago
3- More than 6 months ago, but less than a year ago
4- One year ago or more
5- (Don’t know)
Q6bis - (if yes to Q4) Do you have your own electronic cigarette?
Interviewer: List responses
1- Yes, I have my own
2- No, I share it with someone else
3- No, No, I use other people’s - Go to Q9
4- Other (explain) - Go to Q9
Q7 - ( if yes to Q4) Where did you most recently buy your electronic cigarette?
1- From a shop specialising in electronic cigarettes
2- Online
3- From a tobacconist
4- From a chemist
5- Other (explain)
Q8 - (if yes to Q4) Where did you buy your most recent e-cigarette refill (liquid, cartridge, etc.)?
1- From a shop specialising in electronic cigarettes
2- Online
3- From a tobacconist
4- From a chemist
5- Other (explain)
6- (Not applicable, I use disposable cigarettes)
Q9 - (if yes to Q4) What dose of nicotine do you use in your electronic cigarette?
Interviewer: for zero nicotine, use code 0 and for “don’t know” use code 99
/__/__/
Part II : Tobacco
We are now going to ask you some questions on your tobacco consumption (cigarettes, roll your own tobacco, cigars, cigarillos, pipe, shisha/hookah, etc.) in addition to your use of electronic cigarettes.
Q10 - Do you smoke tobacco, even if only occasionally?
1- Yes
2- No - Go to Q12
Q11 - (if yes to Q10) Did you smoked tobacco within the last 30 days…?
Interviewer: List responses
1- Every day
2- Several times per week
3- Once per week
4- Two to three times in the month
5- Only once in the month
6- (Don’t know)
Q12 - (if no to Q10) Have you ever in your life smoked tobacco regularly or from time to time?
1- Yes, regularly
2- Yes, from time to time
3- (I have only tried it) - Go to Q17
4- (Never) - Go to Q17
Q13 - (if yes to Q12 and yes to Q4) Did you completely stop smoking due to the use of electronic cigarettes ?
1- Yes - Go to Q17
2- No - Go to Q17
Q14 - (if yes to Q10) Where did you last buy your tobacco for your own consumption (pack of cigarettes, roll your own tobacco, cigars, cigarillos, pipe, shisha/hookah, etc.) ?
Interviewer: do not prompt
1- From a tobacconist in France
2- From a tobacconist in a country bordering France (Spain, Andorra, Monaco, Italy, Switzerland, Germany, Luxembourg, Belgium)
3- From a tobacconist in another country
4- From duty-free
5- Online
6- On the street
7- Other
8- (Don’t know)
Part III : Tobacco and electronic cigarette
Q15 - (if yes to Q4 and yes to Q10) Do you currently use…?
Interviewer: List responses
1- Electronic cigarettes most of the time and tobacco sometimes
2- Electronic cigarettes as often as tobacco
3- Tobacco most of the time and electronic cigarettes sometimes
4- (Other)
Q16 - (if yes to Q4 and yes to Q10) In the long term, is your main reason for using electronic cigarettes to…? ONE RESPONSE ONLY
Interviewer: Do not prompt – use codes
1- Stop smoking and vaping completely (tobacco and electronic cigarettes)
2- Reduce my tobacco consumption without stopping smoking tobacco
3- Reduce my tobacco consumption without stopping using electronic cigarettes
4- Substitute electronic cigarettes for tobacco
5- Reduce my spending on tobacco/save money
6- Be able to vape wherever I like (in the office, at home, etc.)
7- Reduce tobacco-related issues (bad breath, yellow teeth, smell of stale smoke, etc.)
8- Reduce the risk of harming my health without stopping smoking tobacco/they are less dangerous for my health
9- No longer be addicted to nicotine/gradually reduce my nicotine dependence using e-cigarettes
10- Other (explain)
11- (Don’t know)
Annex 2. Regulation
At National level
Current French legislation does not consider electronic cigarettes to be a tobacco products, since they do not contain tobacco and do not involve combustion. For this reason, they are not strictly speaking subject to tobacco product regulations, although they are tending towards it. An amendment prohibiting sale to minors was therefore included in the consumer bill (agreement having been reached on this point, this prohibition is set to become law in 2014) and the Ministry of Health wants to ban their use in public places and bars, restaurants etc., despite the fact that no legislative framework has yet been agreed (the Ministry having referred the matter to the Conseil d’État, which has yet to rule). For the moment, only an organisation’s internal regulations can limit their use (as is already the case for some public transport systems, such as RATP, SNCF, Air France, etc.) and the Saint-Lô city council is the only authority to date to have banned use of electronic cigarettes (by administrative order) inside local authority buildings.
Advertising electronic cigarettes is not specifically prohibited, but may fall within the ambit of France’s “Évin” law, which prohibits “any direct or indirect advertising or promotion of tobacco or tobacco products”. Indeed, the hand to mouth behaviour, the vapour released, the possible presence of nicotine, etc. are all so similar to a real cigarette that it is considered that they could possibly encourage smoking. It is this point of law, and more generally, the infringement of the monopoly on tobacco sales that have prompted a French tobacconist to take action for “unfair and illegal competition” against an electronic cigarette retailer located in the vicinity of his business. In December 2013, the Commercial Court of Toulouse found in his favour, relying on Article L.3511-1 of the Code de la Santé Publique [French Public Health Code], which defines tobacco products as “products designed to be smoked […] whether or not they contain tobacco, with the sole exclusion of products designed for medicinal purposes”. The electronic cigarette retailer has appealed this decision, which, if confirmed, could set a precedent and result in the closure of speciality shops in favour of reinstating the tobacconists’ monopoly.
In France, electronic cigarettes are also not considered to be smoking cessation aids or medicinal products, since no manufacturer has claimed as such, which would require them to obtain authorisation to place such products on the market. In the absence of sufficient scientific research on the safety of electronic cigarettes, or on any potentially harmful effects they may have on health, the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS, which became the Agence Nationale de Sécurité du Médicament et des Produits de Santé, the French National Agency for the Safety of Medicines and Health Products, or ANSM) advised against using them in 2011 (http://ansm.sante.fr/var/ansm_site/storage/original/application/6badbfed8724d925b6fafc331da6becc.pdf).
At European and international levels
Discussions to provide a framework for electronic cigarettes at European level fall within the scope of the review of the Tobacco Products Directive 2001/37/EC, which regulates the manufacture, presentation and sale of these products. In December 2012, the European Commission submitted to the European Council and the European Parliament a proposal for a Directive, which was the subject of numerous debates between the various parties involved throughout 2013. Originally planned for September, the proposal to strengthen legislation on tobacco products was examined by the European Parliament in October 2013 (increase in the size of health warnings, prohibition on flavoured cigarettes (except for menthol, for which an extension is granted), list of authorised additives, etc.). The decision to classify electronic cigarettes as tobacco products or medicinal products having not yet been taken, their status as ordinary consumer goods will therefore be retained.
In mid-December, the 28 Member States of the European Union reached agreement on this future “Tobacco Directive” to control tobacco smoking and to better regulate the electronic cigarette market. Consequently, sales of these products to those under 18 years-old will be prohibited, although countries which deem them to be medicinal products may continue to do so (without obliging others, such as France, to do so), the maximum concentration of nicotine will be set at 20 mg/ml (which is a level rarely, if ever, exceeded in points of sale in France) and the European Commission is to submit a report on the health risks associated with using electronic cigarettes within two years.
This agreement is set to be ratified by the European Parliament and at a plenary meeting of the Member States in February/March 2014, before being definitively adopted. After this, each country will have two years to transpose the Directive into domestic law, which means it will not be in force before 2016.
At international level, the World Health Organisation advises against using electronic cigarettes, as long as there is no scientific evidence that they are safe. Only a few countries have imposed a total ban on this product, among them, Brazil, Argentina and Singapore; in others, such as Switzerland and Canada, only zero-nicotine electronic cigarettes can be marketed.
Download PDF version of this translation here

/image%2F0607508%2F20160322%2Fob_177e53_mg-1267.jpg)
/image%2F0607508%2F20160322%2Fob_db3487_voeux-2015b.jpg)



/image%2F0607508%2F20140113%2Fob_fb7c00_nsp-logo03a-290x166.png)
/image%2F0607508%2Fob_358791_farsalinos-web-logo.JPG)
